WEISENTHAL CANCER GROUP
LARRY WEISENTHAL, M.D., PH.D.
Background for the proposed clinical trial of the Gefitinib/Vinorelbine/High Dose Tamoxifen regimen
There are more than 150 drugs with FDA approvals for a cancer indication (Sun, Wei et al. 2017). The number of potential drug combinations is staggering. Curative chemotherapy in cancer virtually always requires combination therapy. The currently popular gene mutation/ molecular marker tests have no ability whatsoever to predict which combinations are actually synergistic. It would be of enormous value to demonstrate that a laboratory technology had the capability of identifying novel drug combinations showing not only laboratory synergy but also clinical efficacy.
Cell culture based tests performed on cells isolated from fresh human tumors (Blom, Nygren et al. 2017) have the ability to detect synergistic drug interactions. Weisenthal in 2007 reported absence of synergy (in fresh tumor cell culture assays) between gefitinib and cisplatin, but strong synergy between gefitinib and vinorelbine (Weisenthal 2007). Subsequently, 4 different clinical trials of the gefitinib + cisplatin combination were considered to be failures (Tsai, Chen et al. 2011). Other investigators also reported synergy between gefitinib and vinorelbine in studies in established cell lines (Erjala, Raitanen et al. 2007; Tsai, Chiu et al. 2012). This synergy was independent of EGFR mutation status (ibid).
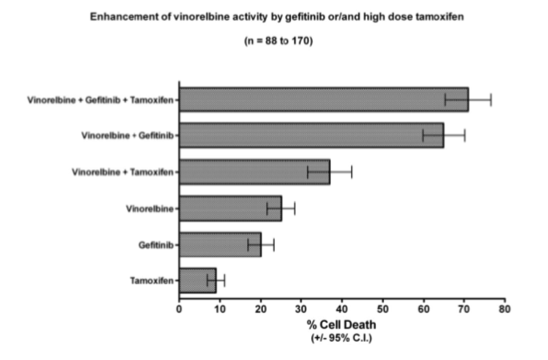
In an attempt to further improve the synergy between gefitinib and vinorelbine, high dose tamoxifen was also tested in a fresh tumor, cell culture assay system (Weisenthal 2011).
Results are displayed in the figure below:
With regard to potential mechanisms of synergy:
1. High dose tamoxifen putatively inhibits p-glycoprotein (Callaghan and Higgins 1995) and/or protein kinase c (O'Brian, Liskamp et al. 1985). P-glycoprotein inhibition would be expected to enhance the activity of vinorelbine, which is a p-glycoprotein substrate (Lagas, Damen et al. 2012).
2. Gefitinib putatively inhibits anti-apoptotic pathways (Sordella, Bell et al. 2004). By inhibiting anti-apoptosis, the apoptosis-inducing effects of vinorelbine could be increased. Gefitinib is also a p-glycoprotein inhibitor (Kitazaki, Oka et al. 2005)
Most recently (2019), Close and colleagues at the University of Pittsburgh published a study in which 2,620 different drug combinations were screened for possible synergy, using the 60 different cell lines in the NCI-60 collection (Close, Wang et al. 2019). Remarkably, the most synergistic of the combinations tested was vinorelbine + gefitinib!
With regard to relevant published clinical trials, there have been no trials published of the three drug combination of vinorelbine + gefitinib + high dose tamoxifen, but there have been several trials of vinorelbine + gefitinib and several trials of vinorelbine (or the related microtubule poison vinblastine) with high dose tamoxifen. In addition, we have a series of case reports with the three drug combination, delivered on a novel and well-tolerated schedule (described below).
Included in the bibliography (see last page) are clinical reports relating to vinorelbine + high dose tamoxifen and vinorelbine + gefitinib. Note particularly the JNCI paper by Trump et al from Duke, with vinblastine + high dose tamoxifen, in which much higher doses of high dose tamoxifen than those used in our regimen (to be described, below) were administered, with modest toxicity (Trump, Smith et al. 1992).
In the sole randomized trial relating to the vinorelbine + gefitinib combination (in heavily pretreated NSCLC), there were superior (1) progression-free survivals and (2) overall survivals in the vinorelbine + gefitinib arm, compared to the single agent gefitinib arm (Chen, Liu et al. 2007). There have been several publications describing cases in which the simple addition of high dose tamoxifen to previously ineffective chemotherapy produced impressive clinical remissions (Yeh and Cheng 1997; Cheng, Yeh et al. 1998; Yang, Cheng et al. 1999; Yeh, Lu et al. 2003).
The clinical trials papers with vinorelbine + gefitinib showed unexpectedly severe myelosuppression (Yoshimura, Nakamura et al. 2004; Pujol, Viens et al. 2006). This actually provides confirmation that this is a clinically synergistic combination. But this toxicity was with the standard continuous dosing schedules of gefitinib (one 250 mg tablet every day). We found the three drug combination to be much less toxic and far more effective on our novel dosing schedule. Our schedule is more rational, given that the reason to combine the three drugs is to exploit synergy between them. Since the rationale for adding gefitinib and tamoxifen to vinorelbine in EGFR non-mutant, estrogen receptor negative cases is simply to enhance the effectiveness of vinorelbine, there is no reason to administer either gefitinib or tamoxifen on days where vinorelbine is not “on board” in the patient.
Our novel three drug regimen is administered on the following schedule (as originally suggested to us by Dwight McKee, M.D.).
1. Weekly vinorelbine (20 to 30 mg/M2)
2. Tamoxifen 40 mg/M2 Q.I.D. X 3 days, beginning one day before vinorelbine. 3. Gefitinib 250 mg B.I.D. X 3 days, beginning one day before vinorelbine.
The above regimen is administered weekly times 3 (D1, D8, D15). Then there is a break until D29, when it is repeated weekly times 3.
Toxicity has consisted primarily of entirely manageable neutropenia. This has required some occasional dose reductions of vinorelbine and occasional vinorelbine-free "holidays" but patients are generally capable of tolerating a full 6 month regimen of treatment, in cases where it is effective. The only other toxicities were mild rash and, with each cycle, initial diarrhea, followed by constipation. We recommend against treating the diarrhea with imodium or other agents, as this just tends to make the subsequent constipation worse.
This regimen has produced durable remissions in a number of patients with otherwise drug resistant adenocarcinomas, including gastric and pancreatic and hepatobilliary carcinomas, in addition to ovarian, breast, and lung adenocarcinomas.
Bibliography
Blom, K., P. Nygren, et al. (2017). "Predictive Value of Ex Vivo Chemosensitivity Assays for Individualized Cancer Chemotherapy: A Meta-Analysis." SLAS Technol 22(3): 306-314. Callaghan, R. and C. F. Higgins (1995). "Interaction of tamoxifen with the multidrug resistance P-glycoprotein." Br J Cancer 71(2): 294-299.
Chen, Y. M., J. M. Liu, et al. (2007). "Phase II randomized study of daily gefitinib treatment alone or with vinorelbine every 2 weeks in patients with adenocarcinoma of the lung who failed at least 2 regimens of chemotherapy." Cancer 109(9): 1821-1828. Cheng, A. L., K. H. Yeh, et al. (1998). "Biochemical modulation of doxorubicin by high-dose tamoxifen in the treatment of advanced hepatocellular carcinoma." Hepatogastroenterology 45(24): 1955-1960.
Close, D. A., A. X. Wang, et al. (2019). "Implementation of the NCI-60 Human Tumor Cell Line Panel to Screen 2260 Cancer Drug Combinations to Generate >3 Million Data Points Used to Populate a Large Matrix of Anti-Neoplastic Agent Combinations (ALMANAC) Database." SLAS Discov 24(3): 242-263.
Erjala, K., M. Raitanen, et al. (2007). "Concurrent use of vinorelbine and gefitinib induces supra-additive effect in head and neck squamous cell carcinoma cell lines." J Cancer Res Clin Oncol 133(3): 169-176.
Kitazaki, T., M. Oka, et al. (2005). "Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells." Lung Cancer 49(3): 337-343.
Lagas, J. S., C. W. Damen, et al. (2012). "P-glycoprotein, multidrug-resistance associated protein 2, Cyp3a, and carboxylesterase affect the oral availability and metabolism of vinorelbine." Mol Pharmacol 82(4): 636-644.
O'Brian, C. A., R. M. Liskamp, et al. (1985). "Inhibition of protein kinase C by tamoxifen." Cancer Res 45(6): 2462-2465.
Pujol, J. L., P. Viens, et al. (2006). "Gefitinib (IRESSA) with vinorelbine or vinorelbine/cisplatin for chemotherapy-naive non-small cell lung cancer patients." J Thorac Oncol 1(5): 417-424.
Sordella, R., D. W. Bell, et al. (2004). "Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways." Science 305(5687): 1163-1167.
Sun, J., Q. Wei, et al. (2017). "A systematic analysis of FDA-approved anticancer drugs." BMC Syst Biol 11(Suppl 5): 87.
Trump, D. L., D. C. Smith, et al. (1992). "High-dose oral tamoxifen, a potential multidrug- resistance-reversal agent: phase I trial in combination with vinblastine." J Natl Cancer Inst 84(23): 1811-1816.
Tsai, C. M., J. T. Chen, et al. (2011). "Antagonism between gefitinib and cisplatin in non-small cell lung cancer cells: why randomized trials failed?" J Thorac Oncol 6(3): 559-568.
Tsai, C. M., C. H. Chiu, et al. (2012). "Gefitinib enhances cytotoxicities of antimicrotubule agents in non-small-cell lung cancer cells exhibiting no sensitizing epidermal growth factor receptor mutation." J Thorac Oncol 7(8): 1218-1227.
Weisenthal, L. M. (2007). Functional profiling with cell culture assays for targeted drug therapy. American Society of Clinical Oncology Gastrointestinal Cancer Symposium, 2007. Orlando FL.
Weisenthal, L. M. (2011). "Differential Staining Cytotoxicity assay: a review." Methods Mol Biol 731: 259-283.
Yang, C. H., A. L. Cheng, et al. (1999). "High dose tamoxifen plus cisplatin and etoposide in the treatment of patients with advanced, inoperable nonsmall cell lung carcinoma." Cancer 86(3): 415-420.
Yeh, K. H. and A. L. Cheng (1997). "High-dose tamoxifen reverses drug resistance to cisplatin and etoposide in a patient with advanced large cell carcinoma of lung." Anticancer Res 17(2B): 1341-1343.
Yeh, K. H., Y. S. Lu, et al. (2003). "High-dose tamoxifen modulates drug resistance to doxorubicin, dacarbazine and ifosfamide in metastatic uterine leiomyosarcoma." Anticancer Res 23(6D): 5133-5137.
Yoshimura, M., S. Nakamura, et al. (2004). "Severe myelotoxicity in a combination of gefitinib and vinorelbine." Lung Cancer 45(1): 121-123.